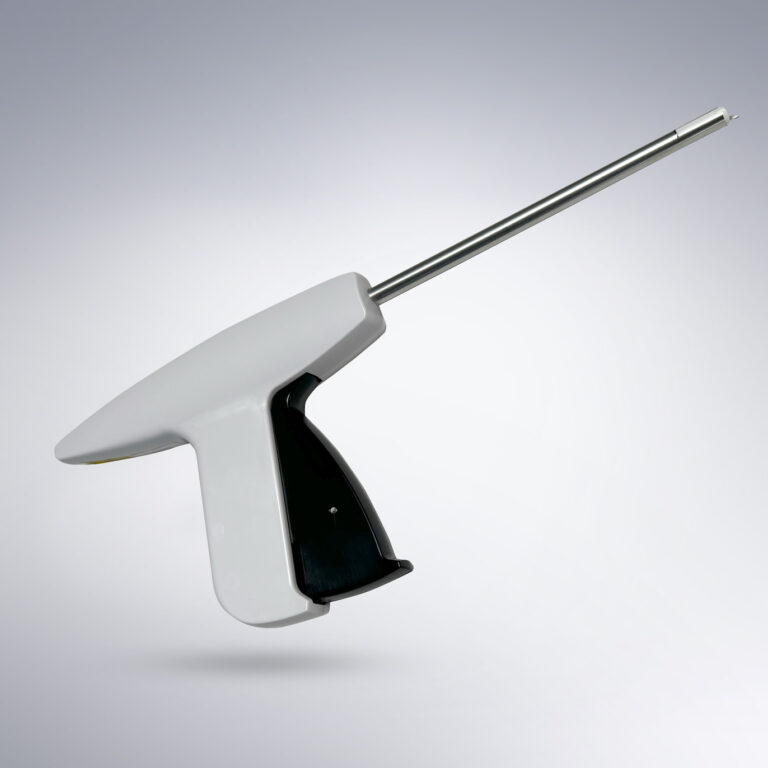
Gaining laparoscopic access to the abdomen is a clinical challenge due to the nature of inserting surgical instruments through small incisions. Laparoscopic entry is a blind procedure, and unfortunately at least 50% of major complications of laparoscopic surgery occur prior to the commencement of the intended surgery (Krishnakumar, 2009). This complication rate has remained stable for the past 25 years (Krishnakumar, 2009). The Hasson technique (open) is considered safer but also more time-consuming with an average access time of 3-10 minutes (Hasson, 1999). The Veress technique is a blind technique that has the advantage of being faster but carries a risk of major vascular injury during insertion (Sangrasi et al, 2011). During Veress needle access, it is critical to generate sufficient counter traction on the abdominal wall to manipulate the extraperitoneal tissue and insert the Veress needle. To address this need, the TauTona Group developed the TauTona Pneumoperitoneum Assist Device, or TPAD, a device which applies suction to the abdominal skin to facilitate easy upward traction during Veress needle insertion, thus improving speed and safety of peritoneal access.
The TPAD is a vacuum-assisted device used to assist in the insertion of a Veress needle when establishing a pneumoperitoneum for laparoscopic surgical procedures. The TPAD could also be used to assist in the placement of the Primary Trocar once the pneumoperitoneum has been achieved.
Use of the TPAD can prevent or reduce trocar and veress needle insertion injuries. These injuries have devastating consequences and are routinely missed or unreported (up to 15%). Additional benefits and advantages of the TPAD are to improve safety, ease of use, and surgeon satisfaction when performing Veress needle and Trocar insertion.
The TPAD has already undergone a first-in-human study (https://clinicaltrials.gov/study/NCT04392635) to assist with Veress Needle insertion, and we also have issued patents with international coverage on the technology (US11690649B2). The TPAD has recently received 510(k) clearance: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K233020